If 54g of A contains 1.5 times of 4 atoms then 18g of B, find the ratio of their atomic weight? - EduRev JEE Question

Find the ratio by mass of the elements present in molecules of hydrogen sulphide (H2S). Given that, H2S molecular wt= 34, Atomicity= 2, Atomic wt= 1

Calculate the ratio between the mass of one atom of hydrogen and mass of one atom of silver - CBSE Class 9 Science - Learn CBSE Forum

Mass numbers of two isotopes of an element differ by 2 (A and A + 2). Average atomic mass is 0.5 than the lower mass number. What could be the ratio of the two isotopes?
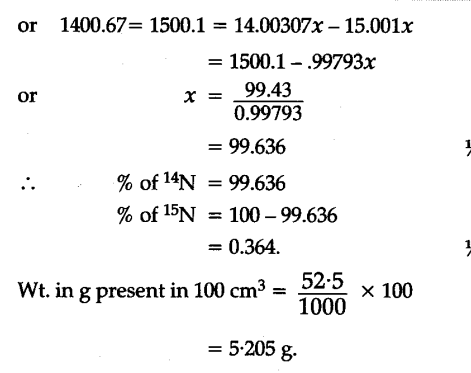
To account for atomic mass of nitrogen as 14.0067, what should be the ratio of 15N and 14N atoms in natural nitrogen? - CBSE Class 11 Chemistry - Learn CBSE Forum

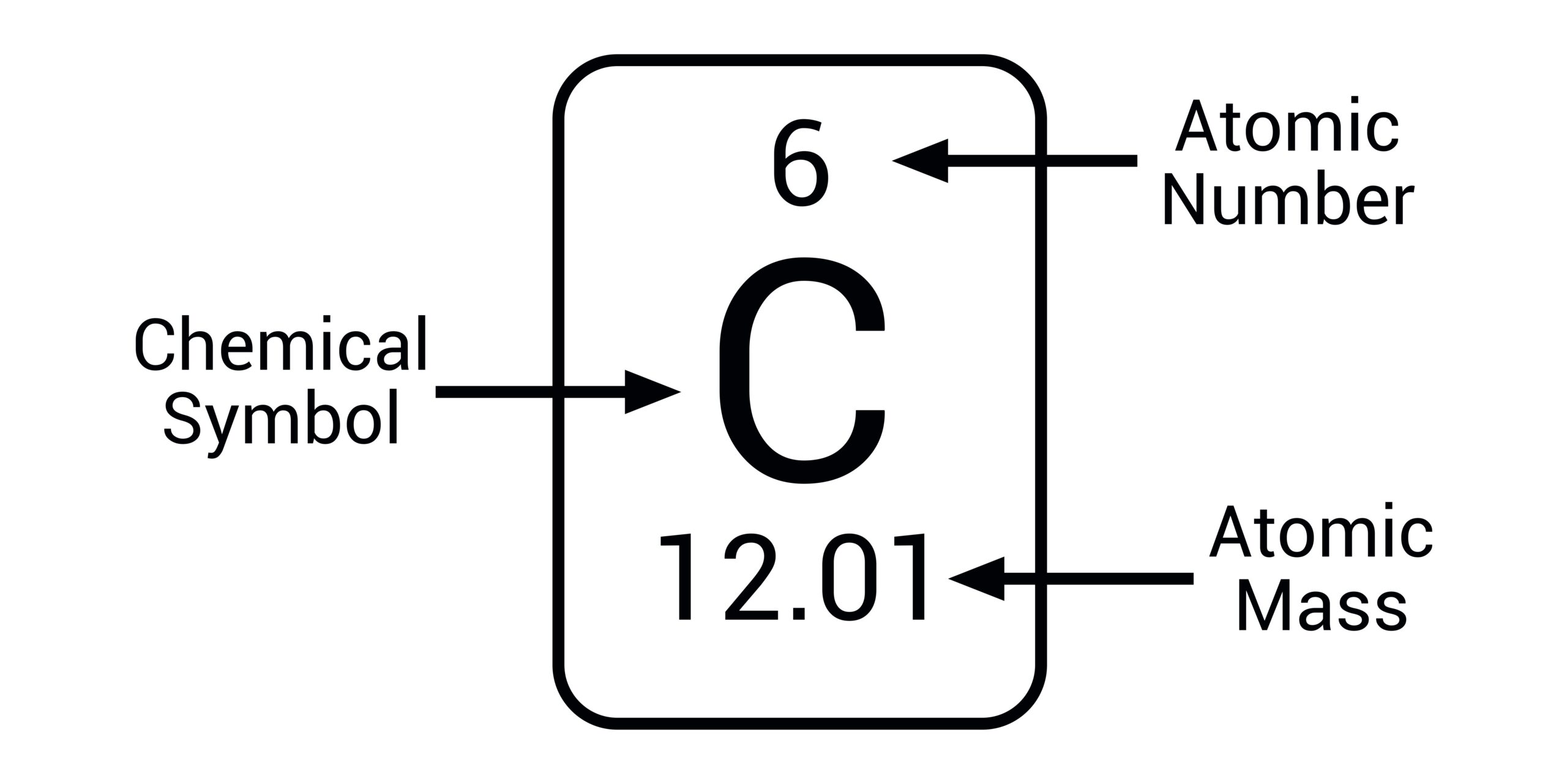
Relative atomic mass (formerly atomic weight): a dimensionless physical quantity, the ratio of the av… | Relative atomic mass, Chemistry education, Atomic mass unit
to account for atomic mass of nitrogen as 14.0067 what should be the ratio of 15^N and 14^N atoms in natural nitrogen. (atomic mass of 14^N = 14.00307 u, 15^N=15.001 u) .

![Kannada] Chlorine (Z =17) has two isotopes with mass numbers 35 and 3 Kannada] Chlorine (Z =17) has two isotopes with mass numbers 35 and 3](https://static.doubtnut.com/ss/web-overlay-thumb/2392125.webp)